Moderna’s Cancer Vaccine Program
Highly successful Covid vaccines have raised the question of ‘why not cancer vaccines?’, a question of great interest and financial contribution for the ALK Positive NSCLC community in recent years. Somewhat under the radar of our members is Moderna, Inc.’s multi-cancer clinical trial for their personalized mRNA vaccine that started in 2017. The trial is open to ALK NSCLC patients after progressing on TKIs, and it is based on the same platform as their approved Covid mRNA vaccine. It is currently in a multiple-arm Phase 1 trial and a randomized, fully enrolled Phase 2 trial.
Previously announced results for head and neck cancer were promising (see below), and further results are anticipated in December of 2022. Moderna’s partner in the trial is pharmaceutical company Merck, as the vaccine is given with pembrolizumab (Keytruda), Merck’s FDA-approved immunotherapy drug. Merck recently paid $250M to Moderna to continue development of their joint treatment, lending optimism to the impending announcement of results in Q4 2022.
Phase 1 Clinical Trial NCT03313778 by Moderna, Inc.; Cancer Vaccine
Study of mRNA-4157 in part in combination with Pembrolizumab (Keytruda) in patients with Unresectable Solid Tumors: Keynote-603 Trial
For ALK+ NSCLC patients, Part B of this trial is a dose escalation study of combination therapy with Moderna’s cancer vaccine mRNA-4157 and checkpoint inhibitor Pembrolizumab (Keytruda) for patients with confirmed unresectable (locally advanced or metastatic) solid malignancies. ALK+ NSCLC patients that are qualified for Part B must have measurable disease and have progressed on approved standard of care treatment for ALK NSCLC. The trial is also open in differing Parts for melanoma, bladder, head and neck, and other cancers. mRNA-4157 is based on the same technology platform that is at the foundation of Moderna’s Covid-19 vaccine.
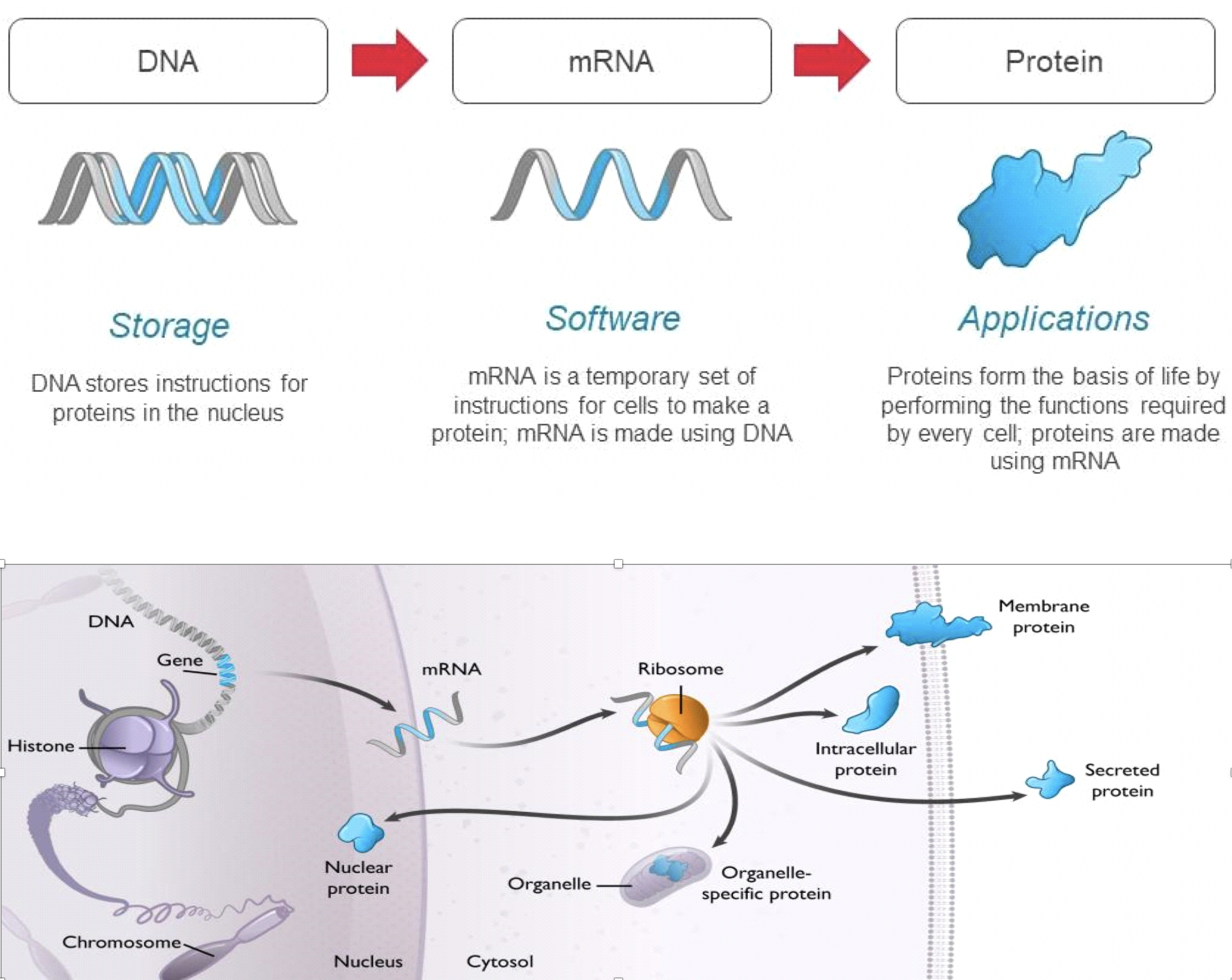
Messenger RNA, or mRNA, is constructed of the same 4-letter “software of life” active in human DNA, but it serves as the transfer agent between the information stored in our genes and the cellular machinery that makes all the proteins required for human biology. Our genes are stored as sequences of DNA which contain the instructions to make specific proteins. DNA serves as a hard drive, safely storing these instructions in the nucleus until they are needed by the cell. When a cell needs to produce a protein, the instructions to make that protein are copied from the DNA to mRNA, which serves as the template for protein production. Each mRNA molecule contains the instructions to produce a specific protein with a distinct function in the body. mRNA transmits those instructions to cellular machinery, called ribosomes, that make copies of the required protein. Every cell uses mRNA to provide real time instructions to make the proteins necessary to drive all aspects of biology, including those relevant to health and disease. Rather than introduce a protein or chemical to the body, Moderna’s vaccines send tailored mRNA into cells to instruct them to produce specific proteins.
The goal of a cancer vaccine is to safely expose the patient’s immune system to tumor-specific antigens (neoantigens) to induce cytotoxic T-cell activation to enable the immune system to elicit a more effective antitumor response. Moderna’s cancer vaccine is focused on the use of mRNA to express (create, generate) neoantigens found in a particular tumor in order to elicit an immune response via T cells that recognize those neoantigens, and therefore the tumor. These neoantigens can either be unique to a patient, as in the case of the company’s personalized cancer vaccine program, or can be related to a driver oncogene found across subsets of patients.
Moderna’s personalized cancer vaccine (PCV) process applies next-generation sequencing to identify gene mutations found in a patient’s cancer cells that can create neoantigens. When tumor cells display a fragment of the neoantigen complexed with human leukocyte antigens (HLA) on the tumor cell surface (an epitope), there is the potential to evoke a vigorous anti-tumor T-cell response. Based on this information, Moderna then generates a personalized mRNA vaccine directed to the many epitopes identified for a specific individual. A rapid cycle time, small-batch manufacturing technique and digital infrastructure can be used to produce each individually manufactured personalized cancer vaccine for the patient rapidly.
The trial has been ongoing for several cancer types since 2017, and in November 2020 the company announced interim data from a 10-patient head and neck squamous cell carcinoma (HNSCC) cohort and a 17-patient colorectal cancer cohort that showed mRNA-4157 in combination with Keytruda is well-tolerated at all levels. Tumor shrinkage responses were produced in head and neck cancer patients, but not in colorectal cancer patients. Overall Response Rate (ORR) in the HNSCC group of patients was 50% (5/10) with two patients achieving a complete response with no detectable disease, and three patients achieving partial response, all of which were ongoing. Median progression free survival (mPFS) was 9.8 months, which compares favorably to the published ORR and mPFS of 14.6% and 2.0 months respectively, for Keytruda monotherapy. Including four patients with stable disease, the Disease Control Rate (DCR) was 90% (9/10). Median duration of response had not been reached. The HNSCC cohort was continuing to recruit, and Moderna decided to expand the size of the current cohort based on the interim data reported. Results for an unknown number of NSCLC patients in this trial, if any to date, are hopefully forthcoming.
The trial across all of its multiple Parts is intended for 143 participants and is recruiting at several sites across the US including Massachusetts General Hospital in Boston and Sarah Cannon Research Institute in Nashville. Contact information and locations are available here:
Moderna, Inc is a publicly held company, listed on the NASDAQ exchange as MRNA, that was originally incorporated in 2009, and is headquartered in Cambridge, MA. It has a diverse development pipeline of 21 programs, of which 10 have entered clinical studies and another three have open INDs (applications for new drug designation with the FDA). Its therapeutics and vaccine development programs span infectious diseases, oncology, cardiovascular diseases, and rare genetic diseases. Financial reports at the end of 2020 describe a very small biotech company with revenues of only $800,000, and a history of development-stage losses. However, Covid-19 revenues in 2021 and the first half of 2022 generated sufficient cash to increase cash balances to $18.1B, providing significant leverage to advance their many programs.
More information on the company can be found here: https://www.modernatx.com/
Author: Jeff Sturm